2022/08/04
Taiwan's AcadeMab develops groundbreaking therapy for COVID-19 Omicron variant with potent neutralizing efficacy
Taiwanese biotech outfit AcadeMab has broken new ground in the development of COVID immunotherapies through the development of a potentially life-saving monoclonal antibodies for COVID-19 patients using single B cell technology, shows the best neutralization ability (IC50 = 11.4 and 4.3 ng/ml) in both Omicron variants BA.1 and BA.2 respectively.
AcadeMab's treatment specifically targets the SARS-CoV-2 Omicron variant – currently the most common strain of COVID infections around the world.
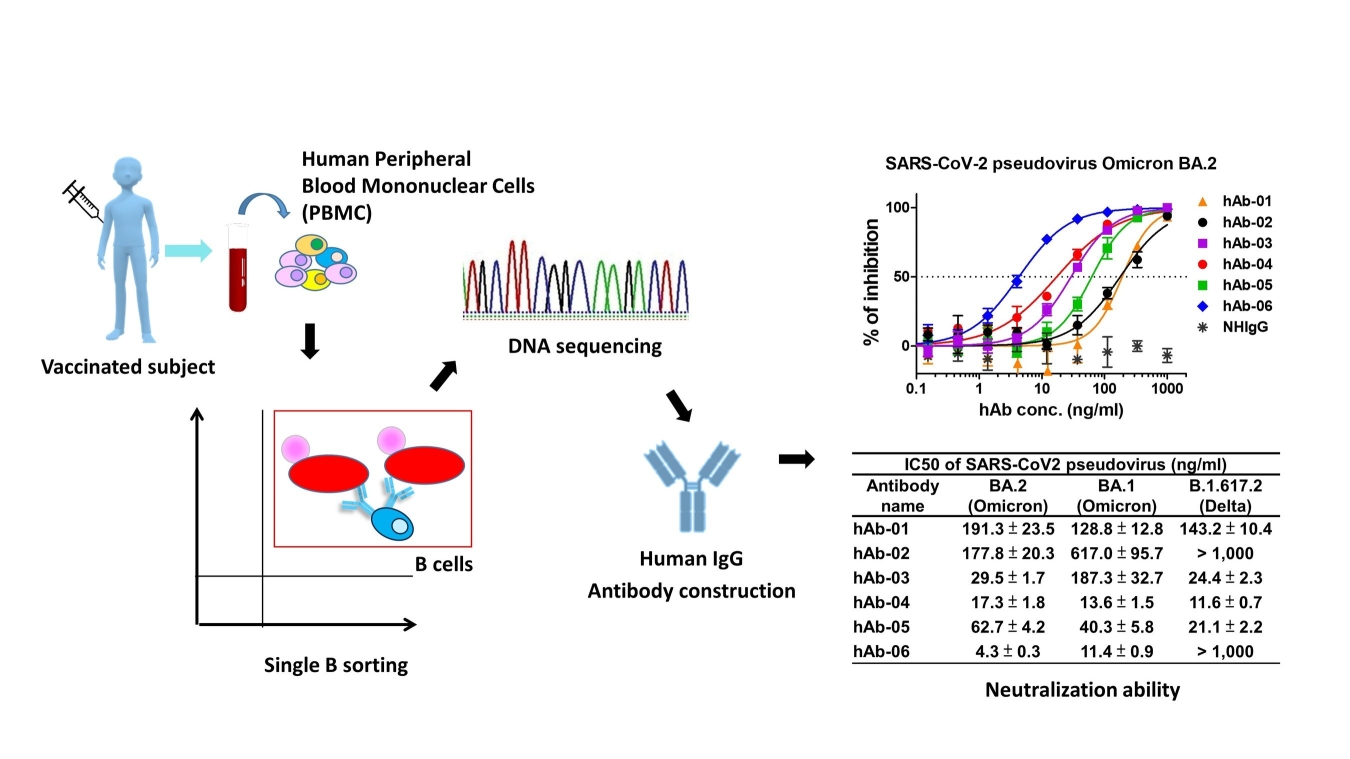
AcadeMab Biomedical Inc., a Taiwan-based biotech, has developed several potent and broad-spectrum COVID-19 therapeutic antibodies for Omicron and Delta variants by single B cell technology from the vaccinated subject at a fast pace.
"In a world ravaged by the COVID-19 pandemic with rapidly mutating variants emerging ever so often, it is a race for the scientific community to develop new therapies in a timely manner."
"We are excited to announce that our research and development efforts have paid off at a fast pace with our single B cell technology that's shown neutralizing efficacy against the world's most common and deadly Omicron variant", shared Juz-Hsiang Chiu, M.D., AcadeMab CEO.
Despite high vaccination rates worldwide, vaccines are only part of the system of treatments to deal with the scourge of COVID-19.
The Omicron variant has been proven to show remarkable resistance to most of the earlier immunotherapies developed, such as Bamlanivimab developed by Eli Lilly and antibody cocktail of Casirivimab and Imdevimab developed by Regeneron pharmaceuticals.
As a result, the Food and Drug Administration (FDA) has revoked or limited the emergency use authorizations (EUAs) for most therapeutic antibodies.
Studies conducted by AcadeMab have found high efficacy of their human monoclonal antibodies against the Omicron variant within 4 months.
In one of their studies, it was found that one human antibody showed the best neutralization ability (IC50 = 11.4 and 4.3 ng/ml) in both Omicron variants BA.1 and BA.2 respectively.
"We've also seen broad-spectrum activity in our treatment against multiple circulating variants of concern (VOCs) announced by the World Health Organisation."
"Aside from its neutralizing efficacy against Omicron, this means it shows great potential to be a therapy of choice for patients infected with other COVID-19 variants aside from Omicron", said Pao-Yin Chiang, Ph.D., the lead scientist of single B cell platform at AcadeMab.
The development of AcadeMab's cutting-edge technology using Single B cell technology will be of immense benefit to a segment of high-risk people who cannot benefit fully from mere vaccination alone.
They include those who are moderately to severely immunocompromised and lack adequate immunity responses despite COVID-19 vaccinations. It is estimated that about 2.7% of adults (or, 7 million) in the U.S population are immunocompromised.
This groundbreaking treatment will also be beneficial to those who have a documented history of severe adverse reactions to existing COVID-19 vaccines or their components, and as such, are unable to be vaccinated.
Parties interested in establishing partnership and learning more about AcadeMab's research may contact:
PR NAME: Miles Yeh, Ph.D., Director of Product Development
CONTACT NUMBER: +886-2789-1212 ext. 810
EMAIL: miles.yeh@academab.com
SOURCE AcadeMab Biomedical Inc.
AcadeMab's treatment specifically targets the SARS-CoV-2 Omicron variant – currently the most common strain of COVID infections around the world.

AcadeMab Biomedical Inc., a Taiwan-based biotech, has developed several potent and broad-spectrum COVID-19 therapeutic antibodies for Omicron and Delta variants by single B cell technology from the vaccinated subject at a fast pace.
"In a world ravaged by the COVID-19 pandemic with rapidly mutating variants emerging ever so often, it is a race for the scientific community to develop new therapies in a timely manner."
"We are excited to announce that our research and development efforts have paid off at a fast pace with our single B cell technology that's shown neutralizing efficacy against the world's most common and deadly Omicron variant", shared Juz-Hsiang Chiu, M.D., AcadeMab CEO.
Despite high vaccination rates worldwide, vaccines are only part of the system of treatments to deal with the scourge of COVID-19.
The Omicron variant has been proven to show remarkable resistance to most of the earlier immunotherapies developed, such as Bamlanivimab developed by Eli Lilly and antibody cocktail of Casirivimab and Imdevimab developed by Regeneron pharmaceuticals.
As a result, the Food and Drug Administration (FDA) has revoked or limited the emergency use authorizations (EUAs) for most therapeutic antibodies.
Studies conducted by AcadeMab have found high efficacy of their human monoclonal antibodies against the Omicron variant within 4 months.
In one of their studies, it was found that one human antibody showed the best neutralization ability (IC50 = 11.4 and 4.3 ng/ml) in both Omicron variants BA.1 and BA.2 respectively.
"We've also seen broad-spectrum activity in our treatment against multiple circulating variants of concern (VOCs) announced by the World Health Organisation."
"Aside from its neutralizing efficacy against Omicron, this means it shows great potential to be a therapy of choice for patients infected with other COVID-19 variants aside from Omicron", said Pao-Yin Chiang, Ph.D., the lead scientist of single B cell platform at AcadeMab.
The development of AcadeMab's cutting-edge technology using Single B cell technology will be of immense benefit to a segment of high-risk people who cannot benefit fully from mere vaccination alone.
They include those who are moderately to severely immunocompromised and lack adequate immunity responses despite COVID-19 vaccinations. It is estimated that about 2.7% of adults (or, 7 million) in the U.S population are immunocompromised.
This groundbreaking treatment will also be beneficial to those who have a documented history of severe adverse reactions to existing COVID-19 vaccines or their components, and as such, are unable to be vaccinated.
Parties interested in establishing partnership and learning more about AcadeMab's research may contact:
PR NAME: Miles Yeh, Ph.D., Director of Product Development
CONTACT NUMBER: +886-2789-1212 ext. 810
EMAIL: miles.yeh@academab.com
SOURCE AcadeMab Biomedical Inc.